臨床検査において、無機リン酸の測定に利用されます。
| 由来 | recombinant E. coli |
|---|---|
| 系統名 | Sucrose : orthophosphate α-D-glucosyltransferase |
| EC 番号 | 2.4.1.7 |
| 反応式 | Sucrose + Orthophosphate →→→ D-Fructose + α-D-Glucose 1-phosphate |

臨床検査において、無機リン酸の測定に利用されます。
| 由来 | recombinant E. coli |
|---|---|
| 系統名 | Sucrose : orthophosphate α-D-glucosyltransferase |
| EC 番号 | 2.4.1.7 |
| 反応式 | Sucrose + Orthophosphate →→→ D-Fructose + α-D-Glucose 1-phosphate |
| Appearance | white lyophilizate |
|---|---|
| Activity | ≧50 U/mg |
| Stabilizer | sucrose |
| Storage condition | below -20℃ |
| Molecular weight | ca. 56 kDa (gel filtration) |
|---|---|
| Structure | monomer of 56 kDa (SDS-PAGE) |
| Isoelectric point | 4.6 |
| Michaelis constant | 3.9×10-2M (sucrose) |
| 6.2×10-3M (phosphate) | |
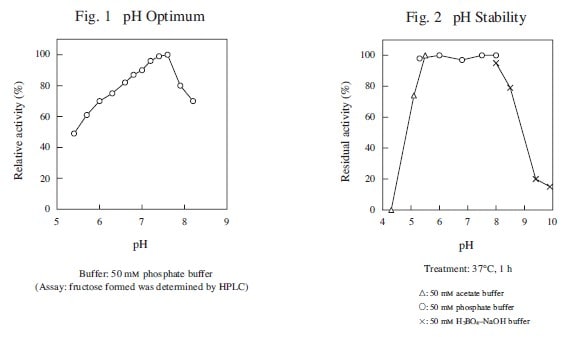
| pH Optimum | 7.5 |
| pH Stability | 5.0–8.0 |
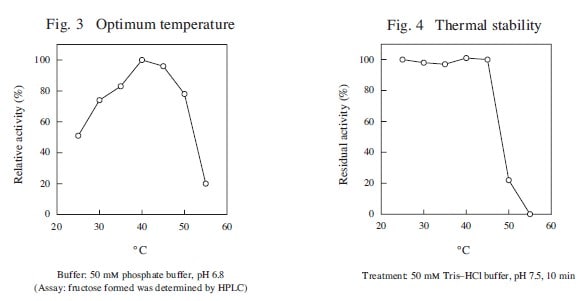
| Optimum temperature | 40℃ |
| Thermal stability | below 45℃ |
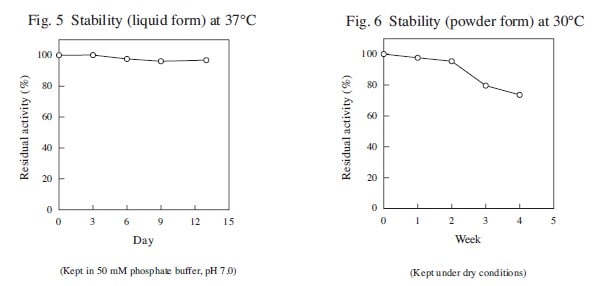
| Stability (liquid form) | stable at 37℃ for at least two weeks |
| Stability (powder form) | stable at 30℃ for at least two weeks |
| Inhibitors | glucose, glucono-1,5-lactone |
| Specificity | sucrose (100), maltose (0), starch (0) |
The enzyme is useful for the determination of inorganic phosphate in clinical analysis.
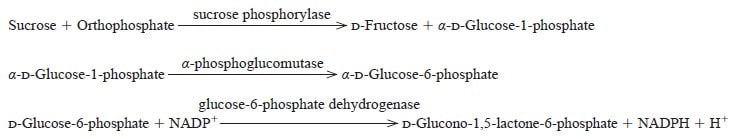
The appearance of NADPH is measured spectrophotometrically at 340 nm.
One unit (U) is defined as the amount of enzyme which produces 1 μmol of NADPH per min at 25℃ and pH 6.8 under the conditions described below.
Sample: dissolve the lyophilized enzyme to a volume activity of 0.8–1.5 U/ml in ice-cold TEA buffer (Reagent A) immediately before measurement.
| 1.5 ml | Potassium phosphate buffer | (Reagent B (a)) |
|---|---|---|
| 1.5 ml | Sucrose solution | (Reagent C) |
| 0.03 ml | EDTA solution | (Reagent D) |
| 0.1 ml | NADP+ solution | (Reagent E) |
| 0.1 ml | G-1,6-P2 solution | (Reagent F) |
| 0.05 ml | MgCl2 solution | (Reagent G) |
| 0.01 ml | α-PGM suspension | (Reagent H) |
| 0.01 ml | G6PDH solution | (Reagent I) |
Activity can be calculated by using the following formula:
![]()
6.2 : Millimolar extinction coefficient of NADPH at 340 nm (cm2/μmol)
df : Dilution factor
C : Content of sucrose phosphorylase preparation in sample (mg/ml)