マルトース及びキシロースに対する反応性が低いFAD依存性グルコース脱水素酵素です。安定性が高く、低温下においても反応性が高いことが特徴です。
| 由来 | recombinant A. sojae |
|---|---|
| 系統名 | D-Glucose : acceptor 1-oxidoreductase |
| EC 番号 | 1.1.5.9 |
| 反応式 | D-Glucose + acceptor →→→ D-Glucono-1,5-lactone + reduced acceptor |

マルトース及びキシロースに対する反応性が低いFAD依存性グルコース脱水素酵素です。安定性が高く、低温下においても反応性が高いことが特徴です。
| 由来 | recombinant A. sojae |
|---|---|
| 系統名 | D-Glucose : acceptor 1-oxidoreductase |
| EC 番号 | 1.1.5.9 |
| 反応式 | D-Glucose + acceptor →→→ D-Glucono-1,5-lactone + reduced acceptor |
| Appearance | yellow lyophilizate |
|---|---|
| Activity | ≧ 475 U/mg |
| Contaminants | NAD glucose dehydrogenase ≺1.0×10-2% |
| Hexokinase ≺1.0×10-2 U/U% | |
| α-Glucosidase ≺1.0×10-2 U/U% | |
| β-Glucosidase ≺1.0×10-2 U/U% | |
| Storage condition | below -20℃ |
| Molecular weight | ca. 90 kDa (SDS-PAGE) |
|---|---|
| Structure | monomer, one mole of FAD per mole of enzyme glycoprotein |
| Michaelis constant | 9.5×10-2M (D-glucose) |
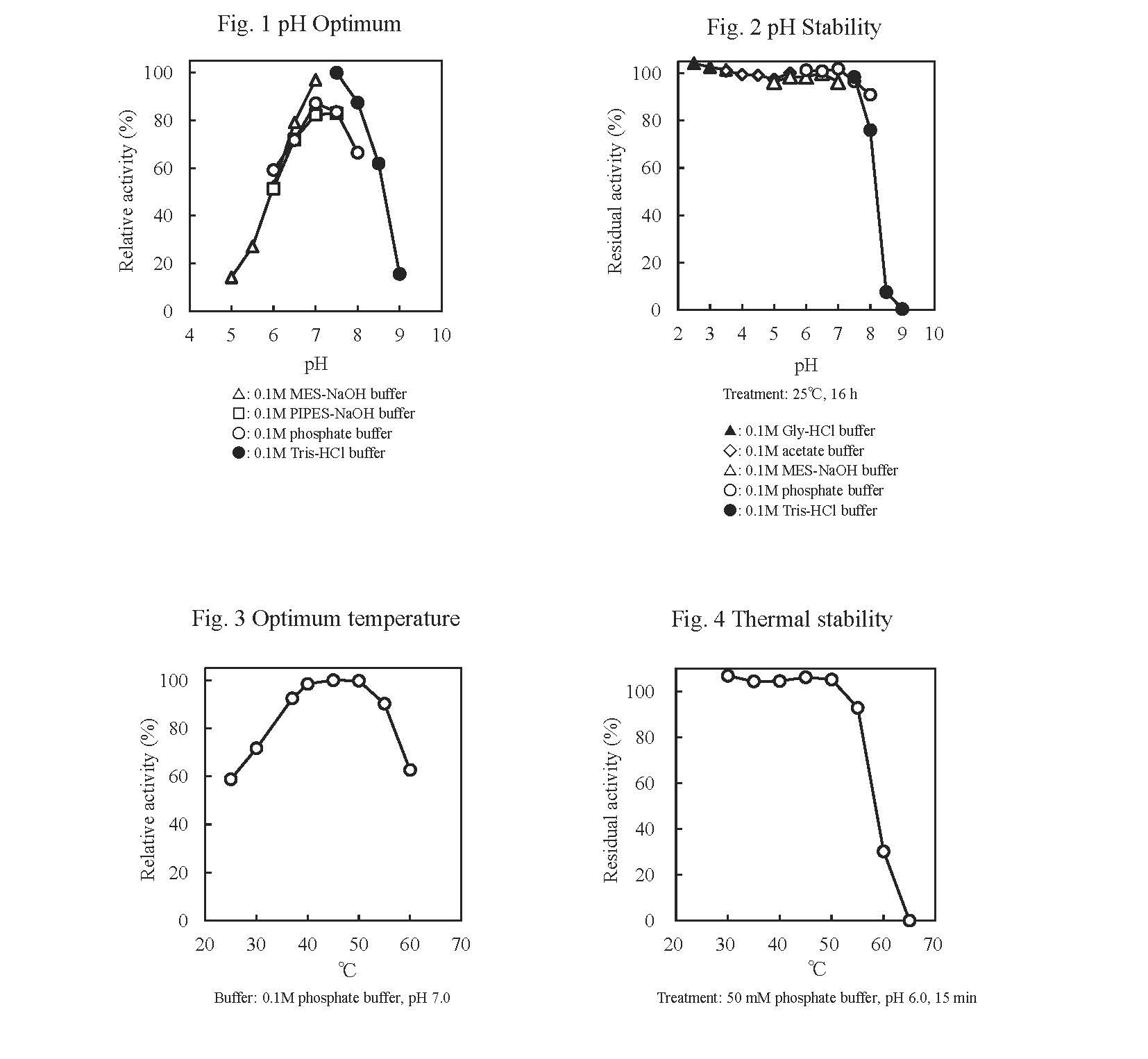
| pH Optimum | 7.0–7.5 (Fig.1) |
| pH Stability | 2.5–7.5 (Fig.2) |
| Optimum temperature | 40–50℃ (Fig.3) |
| Thermal stability | below 50℃ (Fig.4) |
| Inhibitors | Ag+ |
| Specificity | D-glucose (100), maltose (≺1), |
| D-xylose (≺1), D-galactose (≺1) |
FADGDH-AAとフェナジンメトサルフェート(PMS)等の電子メディエータを用いることで、グルコースを比色法や電極法により定量できます。
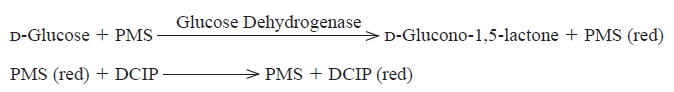
The disappearance of the blue color of DCIP by the reduction is measured spectrophotometrically at 600 nm.
One unit (U) causes the reduction of one micromole of DCIP per minute under the conditions described below.
Sample: dissolve the lyophilized enzyme to final concentration about 0.4 μg/ml with enzyme dilution buffer (Reagent E) immediately before measurement.
Activity can be calculated by using the following formula:
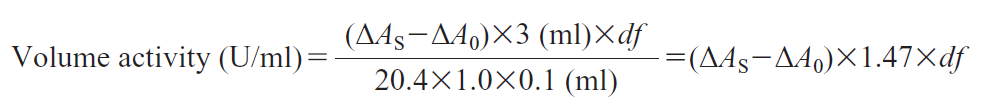
20.4 : Millimolar extinction coefficient of DCIP under the assay condition (cm2/μmol)
1.0 : Light pass length (cm)
df : Dilution factor
Glucose Dehydrogenase (FADGDH-AB)
Glucose Dehydrogenase (FADGDH-AD)