Microbial fermentation
and non-animal origin
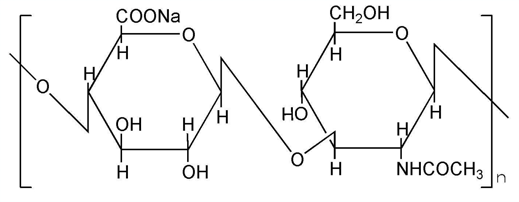
Our sodium hyaluronate is exopolysaccharide fermented from Streptococcus zooepidemicus
which is a kind of lactic acid bacteria.
For API or Excipient

Our sodium hyaluronate is exopolysaccharide fermented from Streptococcus zooepidemicus
which is a kind of lactic acid bacteria.

Our strict quality management system enables us to comply with
GMP control, approved upon PMDA (Pharmaceutical and Medical Device Agency)
and ICH-Q7 (GMP Guide for Active Pharmaceutical Ingredients of ICH)
| Japan : |
GMP approval [12AZ200052]
Master File [217MF10551] |
|---|---|
| US : | Drug Master File for US FDA [12674] |
| EU : | Certificate of Suitability to the European Pharmacopoeia [R0-CEP 2016-247-Rev 00] |
| Korea : | Drug Master File [20180102-96-E-137-42] |
| India : | API Registration Certificate No. RC/BD-002305 |
Complied with each monograph, an official standard,
of the Japanese, European, British, and Korean Pharmacopoeia.
| Japanese Pharmacopoeia | Purified Sodium Hyaluronate |
| European Pharmacopoeia | Sodium Hyaluronate |
| British Pharmacopoeia | Sodium Hyaluronate |
| Korean Pharmacopoeia | Sodium Hyaluronate |

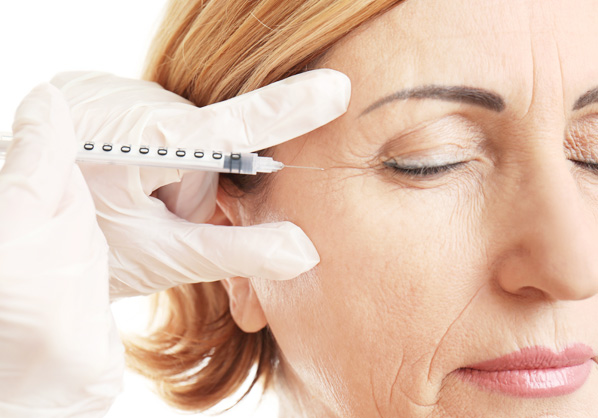
Osteoarthritis
Rheumatoid arthritis
Bone regeneration
Viscosupplementation
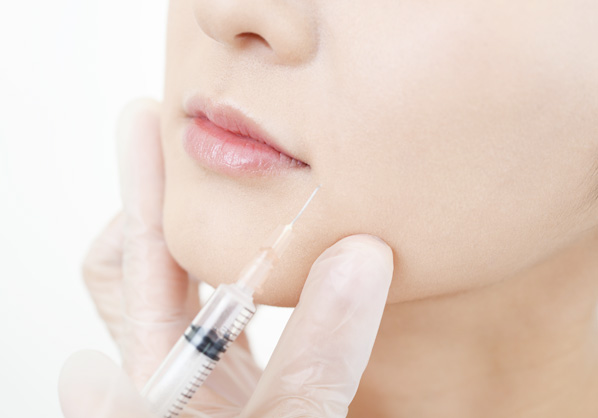
Filling out wrinkles and lines
Lip plumping
Body contouring
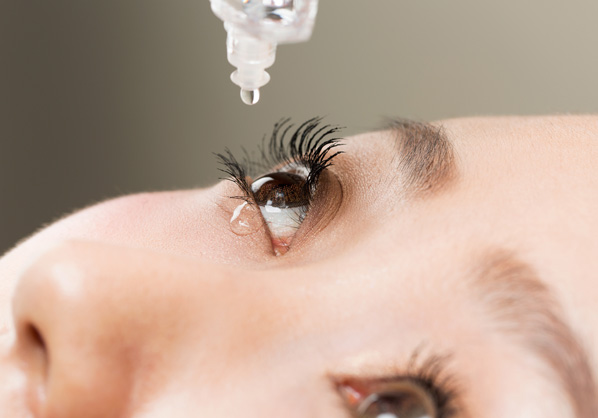
Ophthalmic Viscoelastic Device (OVD)
Eye drop
Contact lens solution
Cataract
Intraocular lens

Skin preparation for external use
Topical skin application
Wound dressing
Medical device coating
Anti - Adhesion
Vesicoureteral and reflux